Hyaluronic acid, as a safe water-based polymer, has applications in many products.
-
Preparation of Hyaluronic Acid
FLUKO EF34 Single Shaft/Coaxial Mixing Series
DescriptionHyaluronic acid, as a kind of safe water-based structural polymer, has applications in many products, including: ophthalmic products, high-end cosmetic injections, tissue filling, etc.
Work Flow1. The sodium hyaluronate powder of the macromolecular chain and the water for injection are uniformly mixed and fully dissolved.
2. Add auxiliary materials and adjust the PH value.
3. Aseptic filling.
Problem1. The density of macromolecular polymer powder is generally about 200kg/m3, which is ultra-light, not resistant to shear, and swells and clumps after contact with water, forming large and small fish eyes, which cannot be effectively handled by conventional equipment.
2. Working in an aseptic environment places harsh requirements on the environment and equipment.
3. High-viscosity system powder dissolving process, the highest viscosity exceeds one million cP.
In an aseptic environment, choosing traditional complete sets of equipment cannot meet production requirements:
Many companies promote common coaxial agitators, but the seal is easily damaged and there are dead ends in cleaning.
Choosing magnetic mixing or single-shaft mixing has the disadvantage of low torque, which can not complete the dissolution of polymer materials. Especially in the high viscosity system of hundreds of thousands to millions of cP, the material rolling is not obvious.
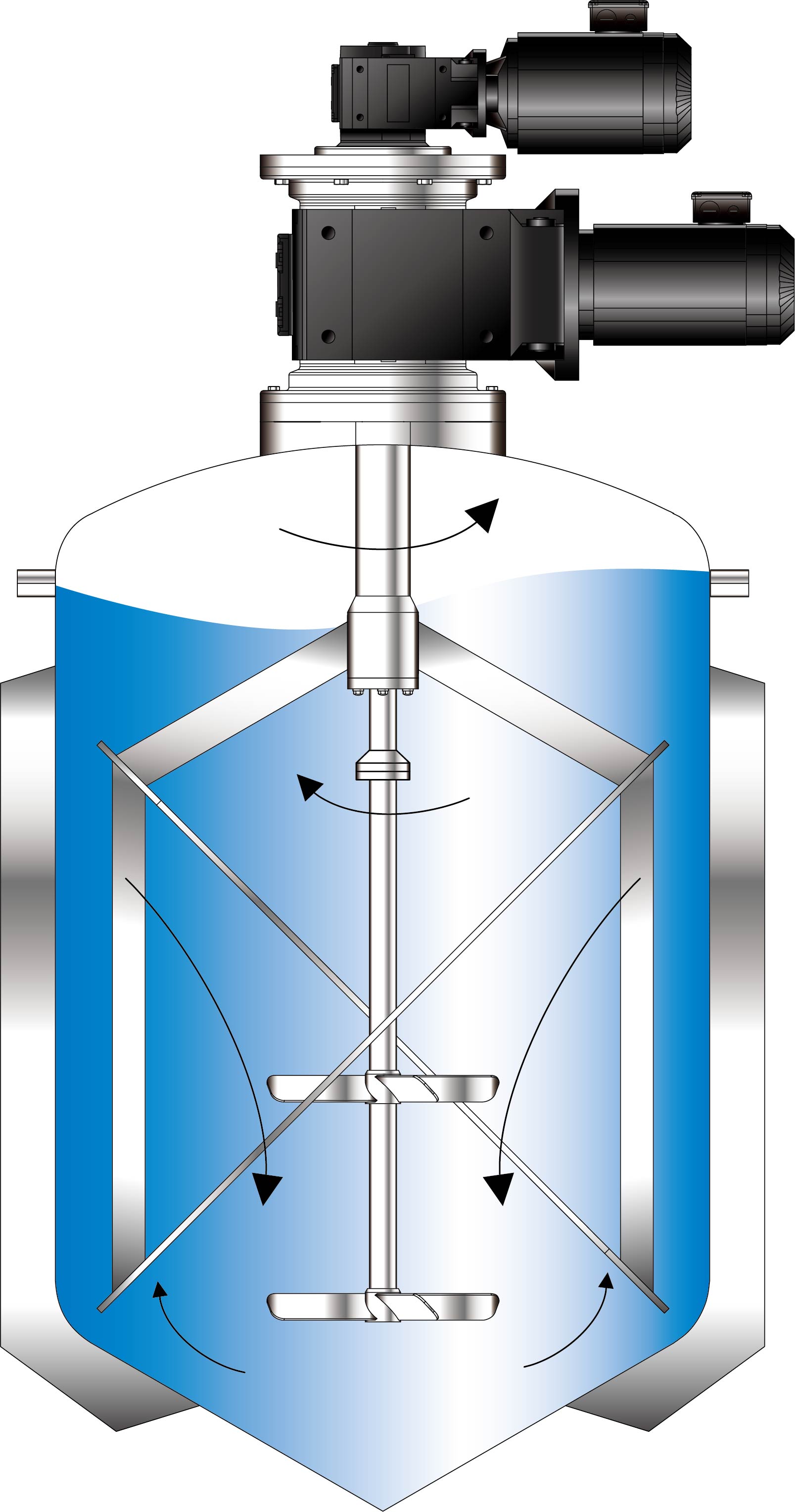
SolutionFLUKO EF34 Special Mixer
 Advantages
Advantages1. FLUKO's EF34 mixer uses computer flow field simulation to mix polymer materials without dead ends.
2. In the mixing process, compared with magnetic fmixing, the efficiency can be significantly improved, and the working time can be reduced by about 50%.
3. It can handle the dissolution process of sodium hyaluronate with molecular chains ranging from ten thousand to one million.
4. It can realize CIP and SIP sterilization process in the field of high-end injection preparations, and meets the requirements of pharmaceutical GMP for no dead ends in the equipment structure.
5. It can realize linear amplification from pilot test to production.